Measuring signaling proteins is critical for modern drug development. Accurate quantification directly impacts the success of Investigational New Drug (IND) and New Drug Application (NDA) submissions. Reliable cytokine assays are essential for elucidating the mechanisms of action, predicting potential toxicity, and monitoring disease progression. However, the dynamic and transient nature of these biomarkers, combined with complex matrix effects, necessitates the selection of high-precision analytical platforms. When properly validated, these assays ensure data integrity, meet rigorous regulatory standards, and accelerate study timelines.
What are Cytokines?
Cytokines are low-molecular-weight proteins that mediate cell-to-cell communication during immune responses. They are produced by various cell types, including macrophages, B lymphocytes, T lymphocytes, and mast cells. These proteins bind to receptors to regulate cell growth, differentiation, and activation.
Key categories of cytokines include:
- Interleukins (ILs): Regulate immune cell activation and division.
- Interferons (IFNs): Primary defense against viral infections.
- Tumor Necrosis Factors (TNFs): Central to systemic inflammation and acute phase reactions.
- Chemokines: Direct the migration of immune cells to infection sites.
Because these proteins often exhibit pleiotropy, where one cytokine has multiple effects, and redundancy, where different cytokines share functions, precise measurement is essential to isolating specific biological pathways.
Importance of Cytokine Assays
Quantifying cytokines is essential for understanding the safety and efficacy of new therapeutics. In preclinical and clinical phases, these measurements provide evidence of how a drug interacts with the immune system.
Primary reasons for conducting these assays include:
- Mechanism of Action: verifying that a drug activates or suppresses the intended immune pathway.
- Toxicity Prediction: Identifying potential adverse events, such as Cytokine Release Syndrome (CRS), before they become clinical risks.
- Pharmacodynamics (PD): Establishing the relationship between drug concentration and biological effect.
Robust data from these assays support decision-making processes for dose selection and helps define safety margins for human trials.
Must Read: Method Validation in LC-MS Mass Spectrometry
Types of Cytokine Assays
Several platforms exist for measuring cytokine levels, each with distinct advantages regarding sensitivity, throughput, and multiplexing capabilities.
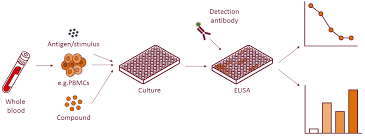
ELISA
The Enzyme-Linked Immunosorbent Assay (ELISA) remains the standard for quantifying single analytes. It uses antibodies designed to bind to a specific cytokine of interest.
- Sensitivity: High, often detecting picogram per milliliter (pg/mL) concentrations.
- Specificity: Excellent, due to the use of matched antibody pairs.
- Utility: Ideal when only one or two cytokines need measurement with high precision.
Multiplex Assays
Multiplex assays enable simultaneous quantification of multiple cytokines from a single small sample volume. Technologies such as Luminex or Meso Scale Discovery (MSD) utilize bead-based or electrochemiluminescence methodologies.
- Efficiency: Generates data on 10, 20, or more analytes at once.
- Sample Conservation: Requires less sample volume compared to running multiple ELISAs.
- Utility: Best for profiling broad immune responses or exploring unknown mechanisms.
Flow Cytometry
Flow cytometry offers a different approach by detecting intracellular cytokines. This method identifies which specific cell populations are producing the cytokines.
- Resolution: Provides single-cell level data.
- Phenotyping: Correlates cytokine production with cell surface markers.
- Utility: Necessary when the cellular source of the cytokine is as important as the concentration.
Applications in Biomarker Discovery
Cytokine Quantification helps identify viable biomarkers. By screening panels of inflammatory mediators, researchers can identify proteins that correlate with specific disease states or therapeutic responses.
In oncology, for instance, specific interferon signatures often predict response to checkpoint inhibitor therapies. In autoimmune disorders, elevated levels of IL-6 or TNF-alpha often serve as indicators for disease flares. Identifying these patterns early allows developers to stratify patient populations more effectively, increasing the probability of clinical trial success.
Disease Monitoring Applications
Once a biomarker is validated, Cytokine Assays transition into tools for longitudinal monitoring. They track how a disease evolves over time or responds to treatment.
Cytokine Release Syndrome (CRS) Monitoring
For therapies such as CAR-T cells or bispecific antibodies, rapid cytokine release can be life-threatening. Assays that provide quick turnaround times for IL-6 and other inflammatory markers are essential for patient safety monitoring.
Chronic Inflammatory Diseases
In conditions like rheumatoid arthritis or psoriasis, regular monitoring of specific cytokines helps clinicians adjust dosing regimens. A reduction in the targeted cytokine’s serum concentration often precedes clinical improvement, serving as an early indicator of efficacy.
Selecting a Cytokine Assay
Choosing the right platform depends on the specific question the study aims to answer. No single platform fits every scenario, and trade-offs between sensitivity and multiplexing are common.
Key selection factors include:
- Analytes of Interest: Are you looking at a broad panel or a specific target?
- Sample Matrix: Is the sample serum, plasma, tissue culture supernatant, or something more complex like cerebrospinal fluid?
- Volume Availability: Preclinical studies with small animals often yield limited sample volumes, necessitating multiplex approaches.
Conclusion
Accurate cytokine measurement is a fundamental component of modern drug development. It links a drug’s mechanism to its clinical outcomes and safety profile. By carefully selecting the appropriate assay platform and ensuring strict adherence to regulatory standards, pharmaceutical companies can secure the data required for successful regulatory interactions. Large Molecule Bioanalysis Solutions support this process by enabling reliable cytokine assessment across development stages. Collaborating with a specialized partner ensures that these critical bioanalytical needs are met with precision and speed.